The atomic nucleus
In the Rutherford-Bohr atomic model the Atoms are constituted by a number of Electrons orbiting around a small Nucleus
containing Proton and Neutron particles. The Atom of each element was classified by a symbol and an atomic number indicating
the Protons to be found in its Nucleus and the equal number of Electrons orbiting around it. The atomic number (1) indicates
the Hydrogen atom, possessing one Electron orbiting a Nucleus of one Proton with a mass that was selected as the unit atomic
weight therefore made equal to one. Every Atom was classified in an atomic table with a number identifying the quantity of
Electrons and Protons forming that particular atom, its mass being in a direct function of the Proton and Neutron sources
packing its Nucleus.
The neutral Nucleon
sources found in the Atom Nucleus are essential to its stability holding the positive Nucleons together.
Experimenting with radioactivity, the discovery was made that a small number
of Atoms in each element can be found possessing a different number of neutral Nucleons within their Nucleus. These Atoms
with similar electrical and chemicals properties, but with a different atomic weight were called Isotopes. J.J. Thomson found
a way to accurately determine the weight of an element by stripping its Atom of one electron, this way creating positive Ions
that could be separated when projected as a beam through a magnetic field.
In 1919 Francis William Aston following in Thomson’s steps perfected a device that could separate and
very accurately measure the mass of each Isotope. With that device Aston discovered that the Nucleons forming the Nucleus
of an Atom were not represented by unit weights adding up to whole numbers, but instead, when a proton and neutron particles
joined in a nucleus, an infinitesimal amount of their mass was in the process transformed into energy.
After this discovery, for practical reasons, Hydrogen 1 was abandoned as
the standard atomic unit weight and at first Oxygen 16 and than Carbon 12 was substituted for it. On the carbon 12 scale the
mass of a free Proton is not equal to one, but actually has a value equal to 1.007825 while the neutron actual mass is 1.008650.
How can this phenomenon be interpreted
in line with the new hypothesis that I made on the substance of matter? In the Atom of Hydrogen, number one on the atomic
table, possessing one Electron revolving around one Proton Nucleus, there is no transformation of any particle into Nucleons.
The atomic weight of H1 is therefore equal to the weight of a Proton 1.007825. We assumed that the transitional velocity of
the Proton at its lowest energy level is provided by the energy contained in the non imploding Nucleon's source. The mass
of a Proton Mo is approximately equal to one GeV. The non imploding mass Mx I found to be equal to 10-6 GeV..
According to the Lorentz equation M (indicating the
increase of mass due to increased velocity) is equal to 1/2 Mo v ^2 / C ^2; a particle increases its mass weight with
its velocity, v ^2= M x C ^2 / 0.5 x Mo. I assumed that if a particle increases its mass when acquiring velocity it must also
release mass with decreasing velocity.
v ^2 = M / 0.5 x Mo x C ^2 vN ^2 =M x C ^2 / o.5 x Mo
In an Electron
ve ^2 =
ve ^2 = 1 / 10^6 GeV / C ^2 ve =C / 1000000 GeV = 3x 10 ^6 Km. x sec
/ 10 ^6
ve = 3 Km. x sec
In a free proton vP^2 = 1 / 10 ^3GeV x Mo x M = 10 ^-3 Gev x Mo xM
Mo = 1GeV M =10 ^-5 GeV C = 1 = 300000 Km / sec
vP = 10 ^-9 GeV ^-2
C= = 300 Km. / sec in natural units =1
The velocity of the source of
a free electron in the field is 1 10000000C. The natural velocity of the source of a free proton or neutron in the field
is 1/10000000 C
When subjected
to electromagnetic or radiating energy, a source will increase its velocity and mass in the ratio demanded by the Lorentz
equation. The energy of mass in a source can be transformed into energy of transitional velocity in a ratio that also can
be deduced from Lorentz equation. In the flrst isotope of Hydrogen, H2, one proton uniting with a neutron packing in a nucleus
must acquire a revolving motion around each other at a determined velocity. In this process the loss in mass will cause an
increased transitional velocity in order to engender a rotation of the two nucleons around each other. The velocity of this
rotation can also be deduced from Lorentz equation. In our proposed atomic model we describe the proton as a source with a
total mass weight equal to 1.007825 and with an infinite life span. A Positron source with a mass weight of 0.0005489 is revolving
around it at a distance from it of 0.5 x 10 ^-20m. By subtracting from the total proton mass, the mass of a positron, we obtain
the mass weight of a nucleon. The mass of a proton is 1.007825, the mass of a neutron is 1.008650 the mass of H2 should be
= 2.016475. On the Aston scale the weight of the isotope H2 is equal = 2.014101. The weight of a nucleon mass is equal to
1.007825 - 0.0005489 = 1.0072761. The total mass weight shed by the H2 nucleus in the transformation is = 0.002374. The mass
lost by a neutron transforming into a nucleon is 1.00865 - 1.0072761 = 0.0013739. The remaining mass left to be shed by the
nucleus as a whole is 0.002374 - 0.001373 = 0.001. Each nucleon must release a mass weight of 0.001 /2 = 0.0005 If M represents
the decrease in mass with the decrease in velocity, Mo is the value of the mass at rest, C is the velocity of light, v is
the additional energy of motion
v ^2 = MC ^2 / 0.5 Mo
vN ^2 =M x C ^2 / o.5 x Mo
If M is equal to the mass loss in one nucleon = 0.0005,
the mass weight of a nucleon at rest Mo = 1.0072761. The velocity of light C = 300000 Km per sec.
vN = 2√10 ^-5 GeV x C / o.7 x 1 GeV = 2√10 ^-4 GeV x C /2.67
vN =-10 ^ -2 GeV x C / 1.42 = 0.01 / 2,67 x C =0.01.42 x C = 0.14
x C

In the atom of the isotope H2 the velocity of revolution of two nucleons around
each other must acquire a velocity of 0.031 x C shedding a mass equal to 0.0005 and maintain their nucleus requisites in mass.
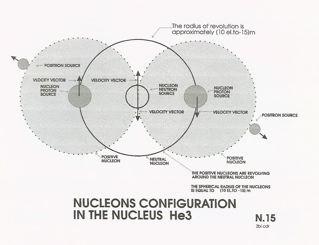
In following the same line of thought, we will now consider the isotope of Helium, He3, which has a nucleus with three nucleons
that we assume to have a configuration as shown in sketch N.15. The nucleus of He3 is made by two protons and one neutron
adding up to a mass weight of
1007825 + 1007825 + 1008650 = 3.0243
On the Aston mass spectrograph the mass weight of He3 is equal to 3.0160. The total mass shed into energy by the
nucleus in the nuclear transformation is 0.0083. The mass lost by the neutron transforming into a nucleon is 0.001373. There
must be (in order to complete the process of forming a nucleus) an additional mass weight loss by the nucleus equal to 0.0083
- 0.00137 = 0.007. The mass weight lost by each nucleon is then 0.007 / 3 = 0.002333. If we are assuming, as shown in our
sketch N15, that the nucleons in He3 have a configuration consisting of one neutral nucleon sandwiched between two positive
nucleons, the central neutral source is under the influence of the two positive sources acting on its velocity vector through
their intensity impulses. Since the sources of the positive nucleons vectors are directed in opposite directions, their action
must cancel, leaving the neutral nucleon free to proceed at its original transitional velocity.
In considering again the Lorentz formula
M = 1/2 x Mo v2 / C2 vN = velocity of Nucleon N
vN= 2 x MC2 / Mo = 2 x 10 ^-5 GeV C ^2 / C ^2 = 2 x 10 ^-5 C
If we make M equal to the mass
lost by one nucleon = 0.002333.
Mo the
value of the nucleon mass at rest = 1.0072761.
C
the velocity of light = 300000 Km per sec equal to 1 in natural units.
v the increase in velocity
v2 = 2 x 0.002333 x 1.007276 x C2 / 1.0072761
v2 = 2 x 0.002333 x C2 = 0.004666 x C2
v = √0.004666
x C2 = 0.068 C
In
the atom He3 the positive nucleons will revolve around the neutral nucleon at velocity equal to 0.068 C. Although the arrangement
of the nucleons in the nucleus of an atom becomes more complicated with the increase in its atomic weight making our assumptions
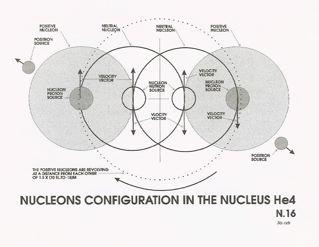
more tentative, we will try to describe the atom of Helium 4. (See sketch N18) We assume that the atom of He4 is composed
(as indicated by its atomic number) by four nucleons, two of them being of a positive nature and two of neutral nature. The
sum of two protons plus two neutrons adds up to a mass weight of 4.03295. On the Aston mass spectrograph the weight of He4
is shown to be 4.00260. The resulting mass weight loss by the nucleus is equal to 0.03035.
The mass of 2 neutrons is 2 x 1.008650 = 2.0173. The mass of 2 nucleons is 2 x 1.007276 = 2.0145. The total masses
lost in the transformation of the two neutrons are 2.0173 - 2.0145 = 0.0028. The mass to be lost by 4 nucleons is 0.030 -
0.0028 = 0.027. The mass lost by each nucleon is 0.027 / 4 = 0.0068
v2 = 2 x 0.0068 x 1.007276 x C2 / 1.007276 = 0.0136 x C2
v = 0.1l6 x C
The value of the additional velocity for He4 is equal
to 0.116 x C. Assuming that the nucleus of Helium 4 is composed of 2 neutral nucleons sandwiched between 2 positive nucleons,
these last must revolve around each other at a distance equal to 1.5 x 10-15 at a velocity equal to 0.116 x C over the original. The electrons and positrons in the atom are not affected
by changes in the self-contained energy of the nucleons, but changes in their velocity are transmitted to the positron sources
that are imprisoned in the nucleus by its implosion waves and are through them transferred to the electrons.
At its lower energy status the natural transitional
velocity of a proton and of a single atom of hydrogen is 0.0044 x C.
The velocity of revolution in the nucleus of the isotope H2 is 0.031 x C.
The velocity of revolution in the nucleus of the isotope H3 is 0.068 x C.
The velocity of revolution in the nucleus of Helium
H4 is 0.116 x C.